full electron configuration for sulfur|ground state electron configuration for chlorine : Bacolod Mar 23, 2023 No caso de utilizadores atuais que convidam amigos para o Serviço HP Instant Ink, apenas os convidados qualificados (conforme definido nos Termos e Condições do Serviço HP Instant Ink) são elegíveis para o Serviço gratuito oferecido pelo Programa Convide um Amigo (Refer-a-Friend), tendo como base o plano do Serviço então escolhido.Description: Watch tongue fucking riley reyes and pressley carter rim buttholes in maricar reyes and hyden kho salas together with other porn videos like fucked in the bathtub of her stepbrother and sci fi female android fucks an alien in the surgery room in the space station. - maricar reyes and hyden kho salas video 2
PH0 · show electron dot diagram for k
PH1 · pb 2+ electron configuration
PH2 · magnesium 12 electron configuration
PH3 · ground state electron configuration for chlorine
PH4 · give the full electronic configuration for phosphorus
PH5 · electron configuration for nitrogen
PH6 · complete the electron configuration for s
PH7 · complete the electron configuration for cl
PH8 · Iba pa
A huge collection of free porn comics for adults. Read The Fear Comics online for free at erofus.com
full electron configuration for sulfur*******In writing the electron configuration for Sulfur the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for sulfur go in the 2s orbital. The next six electrons will go in the 2p orbital. The p orbital can hold up to six electrons.
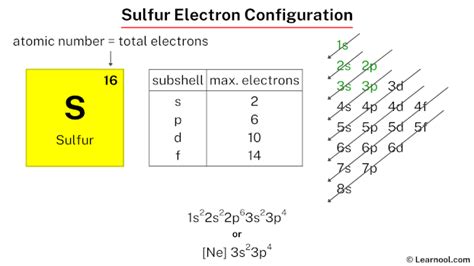
Mar 23, 2023 Aufbau principle. First, find electrons of sulfur atom. Periodic table | Image: Learnool. The atomic number of sulfur represents the total number of electrons of sulfur. Since the atomic number of sulfur is 16, . In order to write the S electron configuration we first need to know t.
Ground State Electron Configuration of Sulfur. when we the electron configuration of Sulfur the first two electrons go in the 1s orbital. As 1s only hold two electrons and the next two electrons for . Find the full electronic configuration and valence electrons of any periodic element using this electron configuration calculator.
What is the full electron configuration for sulfur? The full electron configuration for sulfur is 1s² 2s² 2p⁶ 3s² 3p⁴. This configuration shows the distribution of .
Electron configuration for sulfur. The history of Sulfur. Periodic table history. Identifiers. List of unique identifiers for Sulfur in various chemical registry databases. Sulfur is a .
Sulfur atoms have 16 electrons and the shell structure is 2.8.6. The ground state electron configuration of ground state gaseous neutral sulfur is [ Ne ]. 3s2. 3p4 and the term .
ground state electron configuration for chlorine The full electron configuration of sulfur can be represented as 1s 2 2s 2 2p 6 3s 2 3p 4. This notation provides a detailed breakdown of the number of electrons in each energy level and sublevel of the atom. It helps us understand the arrangement of electrons and their distribution across different orbitals.
The Shorthand electron configuration (or Noble gas configuration) as well as Full electron configuration is also mentioned in the table. Atomic no. Element Shorthand Electron Configuration .
The electron configuration for phosphorus is 1s 2 2s 2 2p6 3 s2 3p3 and the orbital diagram is drawn below. 1.4: Electron Configurations and Electronic Orbital Diagrams (Review) is shared under a license and was authored, remixed, and/or curated by LibreTexts. The electron configuration of an atom indicates the number of valence . There are six valence electrons in the outer shell of the Sulfur. Ground State Electron Configuration of Sulfur. when we the electron configuration of Sulfur the first two electrons go in the 1s orbital. As 1s only hold two electrons and the next two electrons for sulfur goes to the 2s orbital. Hence the S electron configuration is 1s 2 . To write the orbital diagram for the Sulfur atom (S) first we need to write the electron configuration for just S. To do that we need to find the number of e.Sulfur. 16. 32.06. Glossary. GroupA vertical column in the periodic table. Members of a group typically have similar properties and electron configurations in their outer shell. PeriodA horizontal row in the periodic table. The atomic number of each element increases by one, reading from left to right.The electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon (18 electrons) are analogous in the electron configurations of their outer shells to their corresponding family members carbon, nitrogen, oxygen, fluorine, and neon, respectively, except that the principal quantum .
Give the full electron configuration for sulfur. electron configuration: Your solution’s ready to go! Our expert help has broken down your problem into an easy-to-learn solution you can count on.Give the full electron configuration for sulfur. electron configuration: Your solution’s ready to go! Our expert help has broken down your problem into an easy-to-learn solution you can count on.full electron configuration for sulfur ground state electron configuration for chlorineGive the full electron configuration for sulfur. electron configuration: Your solution’s ready to go! Our expert help has broken down your problem into an easy-to-learn solution you can count on. In this video we will write the electron configuration for S 2-, the Sulfide ion. We’ll also look at why Sulfur forms 2- ion and how the electron configurati.Electron configuration 3s 2 3p 4: Electrons per shell: 2, 8, 6: . both full Latin spelling variants sulfur and sulphur became common in English. . They use sulfur as the electron acceptor, and reduce various oxidized sulfur compounds back into sulfide, often into hydrogen sulfide. They can grow on other partially oxidized sulfur compounds .
A single Sulfur atom has 16 protons and 16 electrons, but how do we know where Sulfur puts its . Let's find the ground state electron configuration of Sulfur! Referring to Figure 3.4.3 3.4. 3 or Figure 3.4.4 3.4. 4, we would expect to find the electron in the 1 s orbital. By convention, the ms = +12 m s = + 1 2 value is usually filled first. The electron configuration and the orbital diagram are: Following hydrogen is the noble gas helium, which has an atomic number of 2. Again, consider sulfur, S, which, based on its electron configuration, has 6 valence electrons. Sulfur is located in the 16 th column of the periodic table. However, the "A/B System" is used to label .

Electronic configuration for Sulfur (S) The atomic number of Sulfur is 16. Sulfur belongs to group 16 of the Modern periodic table. In the periodic table, Sulfur is placed in the third period. For the electronic configuration of an element there are three important rules which must be followed: Aufbau Principle Pauli-exclusion Principle Hund's . Example 2.6.1 2.6. 1. Write the electron configuration for nitrogen. Solution. In order to write an electron configuration for nitrogen (N), the total number of electrons in an atom of nitrogen must first be determined. Since nitrogen has an atomic number of 7, an atom of nitrogen contains 7 protons and 7 electrons.
Since 1s can only hold two electrons the next 2 electrons for Chlorine go in the 2s orbital. The next six electrons will go in the 2p orbital. The p orbital can hold up to six electrons. We'll put six in the 2p orbital and then put the next two electrons in the 3s. Since the 3s if now full we'll move to the 3p where we'll place the remaining .
full electron configuration for sulfurIn writing the electron configuration for Potassium the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Potassium go in the 2s orbital. The next six electrons will go in the 2p orbital. The p orbital can hold up to six electrons. We'll put six in the 2p orbital and then put the next . Sulfur(S) excited state electron configuration and orbital diagram. Therefore, the electron configuration of sulfur(S**) in an excited state will be 1s 2 2s 2 2p 6 3s 1 3p x 1 3p y 1 3p z 1 3d xy 1 3d yz 1. This electron configuration shows that the last shell of the sulfur atom has six unpaired electrons. So the valency of sulfur is 6.
This caused Blue's Machoke to evolve, and Blue took the opportunity to teach Red's Pokémon more exotic, varied, and useful moves. Red's training on Blue's Pokémon made them more playful and .
full electron configuration for sulfur|ground state electron configuration for chlorine